Original Periodic Table Dmitri Mendeleev Mendeleev First Periodic Table Page 1 Line 17qq Com
Mendeleev's periodic table was a significant breakthrough in chemistry, providing a systematic way to organize the elements and predict their properties. For example, if you have a sample of sodium and understand its properties, you know the properties of lithium (above sodium on the table) and potassium (below sodium) without ever seeing them.
PPT The Periodic Table PowerPoint Presentation, free download ID6737157
. Mendeleev published his first periodic table of the elements in 1869. Features of Mendeleev's tables Mendeleev arranged the elements in order of increasing relative atomic mass . When.
The Periodic Table Chemistry Quizizz
Ann E. Robinson The United Nations declared 2019 to be the International Year of the Periodic Table, celebrating the 150th anniversary of the discovery of the periodic law. Early in 1869, Russian chemist Dmitri Mendeleev was in a predicament many people are familiar with—he was facing a deadline.

PPT The Periodic Table of Elements PowerPoint Presentation, free download ID3324266
Building the Periodic Table of the Elements Dmitri Mendeleev in 1897, public domain By Michelle Feder Dmitri Mendeleev, a Russian chemist and teacher, devised the periodic table — a comprehensive system for classifying the chemical elements. Organizing Matter

PPT 2.2 The Periodic table and Chemical Properties PowerPoint Presentation ID1993170
Mendeleev's periodic table gained wide acceptance with the scientific community and earned him credit as the discoverer of the periodic law. Element number 101, synthesized in 1955, is named mendelevium after the founder of the periodic table. It was, however, several years after Mendeleev died before the several discrepancies with the atomic.
How Dmitri Mendeleev Developed The Periodic Table YouTube
periodic table, now turning 150 years old. A century and a half ago, a Russian chemistry professor published a classification of all the known elements, organized by atomic weight. Today, the system that he created for his students — plus some updates and including about twice as many elements — is found in chemistry classrooms around the.

Biography of Dmitri Mendeleev, Inventor of the Periodic Table Dmitri mendeleev, Periodic table
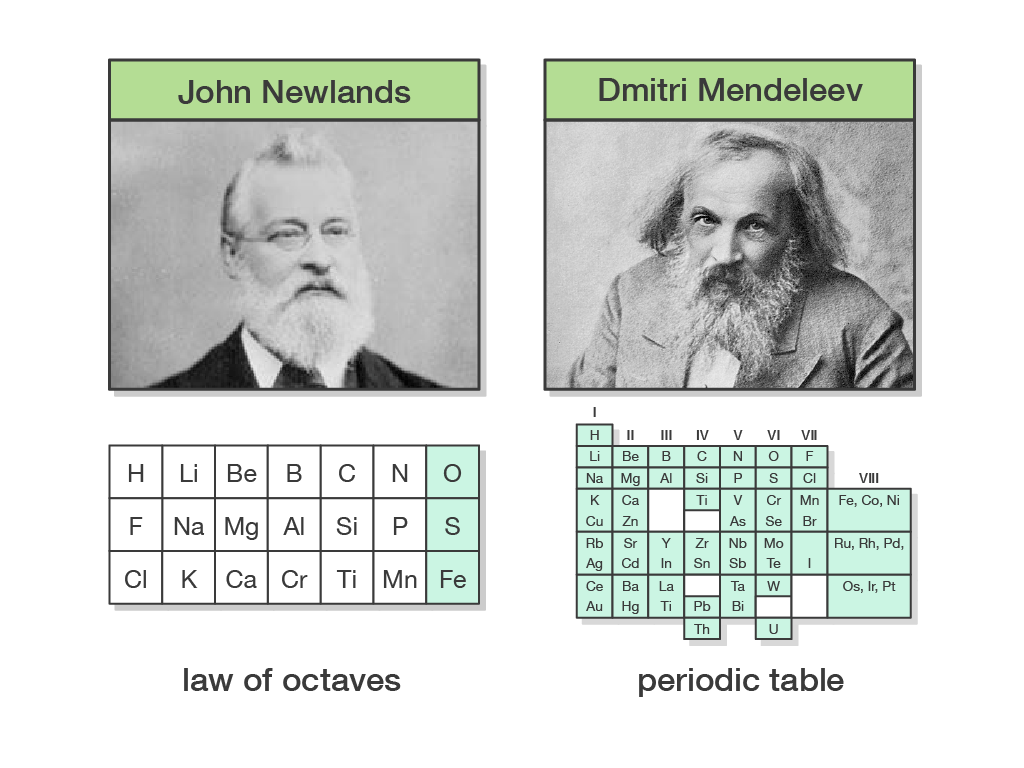
1. Similar elements were grouped together. 2. Elements were arranged in order of increasing atomic masses. People also ask What is the periodic table of the elements? Physical and Chemical Properties of Group 1 Elements Physical and Chemical Properties of Group 18 Elements Physical and Chemical Properties of Group 17 Elements

Discovery of the Periodic Table History Today
Mendeleev published his table, which he called a "periodic system," in 1869. It included all 56 elements then known, and if you squint, it has a somewhat similar shape to the periodic table we.

150 years ago, the periodic table began with one chemist’s vision Science News
Table formation Ask most chemists who discovered the periodic table and you will almost certainly get the answer Dmitri Mendeleev. Certainly Mendeleev was the first to publish a version of the table that we would recognise today, but does he deserve all the credit?
.PNG)
Ñëàéä 59 Chapter Outline • The Periodic Table Used to organize the elements by recurring
periodic trend . He then arranged the elements by putting those with similar properties below each other into groups. To make his classification work, Mendeleev made a few changes to his.

PPT Unit 27 Introduction to periodic table PowerPoint Presentation, free download ID1587606
Many scientists devised periodic systems in the 1860s, but Dmitri Mendeleev is today recognized as the father of the periodic table. How did this Russian provincial come to possess one of the most famous names in science? by Michal Meyer

Modern Periodic Table Dmitri Mendeleev State Mendeleev's periodic law What is Mendeleev
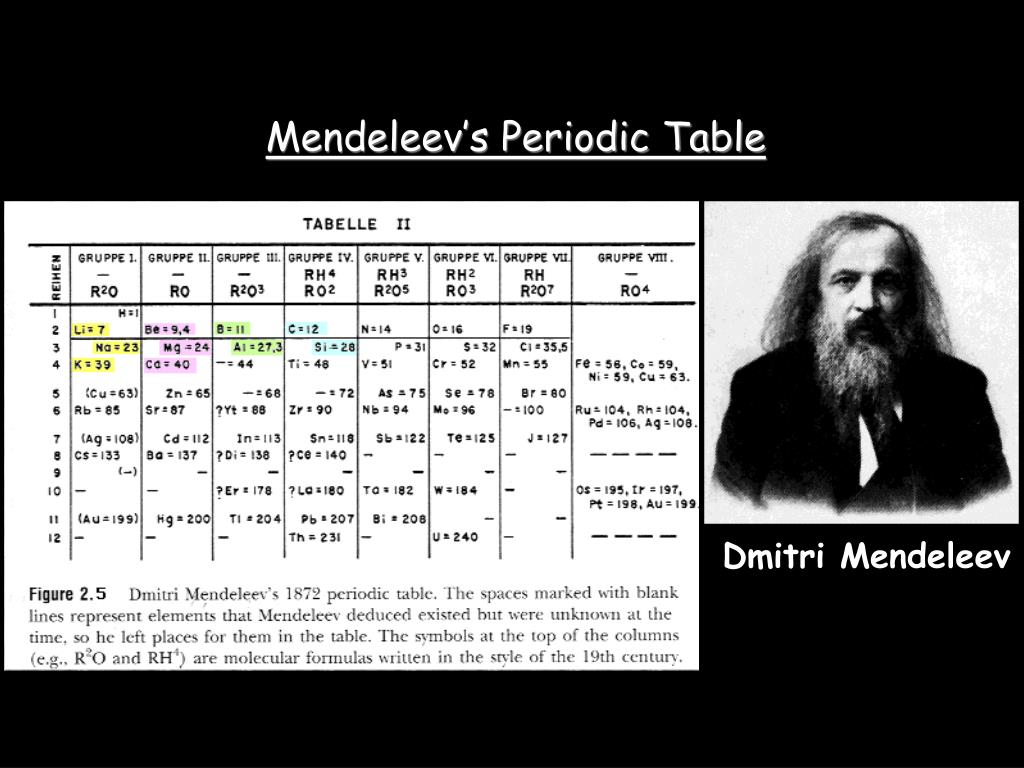
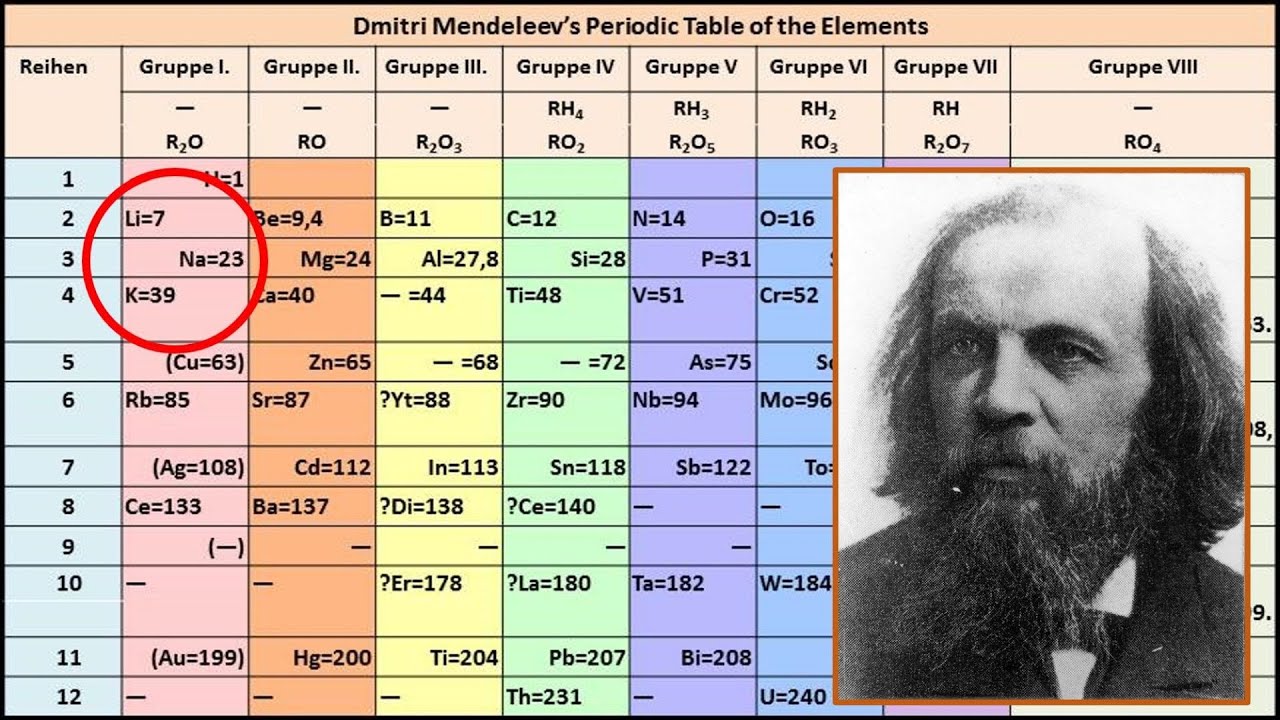
The groups in Mendeleev's table are determined by how many oxygen or hydrogen atoms are needed to form compounds with each element. For example, in Group I, two atoms of hydrogen, lithium, Li, sodium, Na, and potassium form compounds with one atom of oxygen. In Group VII, one atom of fluorine, F, chlorine, Cl, and bromine, Br, react with one.

Dmitri Mendeleev profile Google Doodle marks the 182nd birthday of the man who invented the
Dmitri Mendeleev Russian scientist Cite External Websites Also known as: Dmitri Ivanovich Mendeleev, Dmitry Ivanovich Mendeleyev Written by Bernadette Bensaude-Vincent Professor of the history and philosophy of science, University of Paris X Nanterre, France. Bernadette Bensaude-Vincent Fact-checked by The Editors of Encyclopaedia Britannica
Listen to music albums featuring Mendeleev's Periodic Table by History Talk from Origins online
In Mendeleev's periodic table, elements were arranged on the basis of the fundamental property, atomic mass, and chemical properties. During Mendeleev's work, only 63 elements were known. After studying the properties of every element, Mendeleev found that the properties of elements were related to atomic mass in a periodic way.
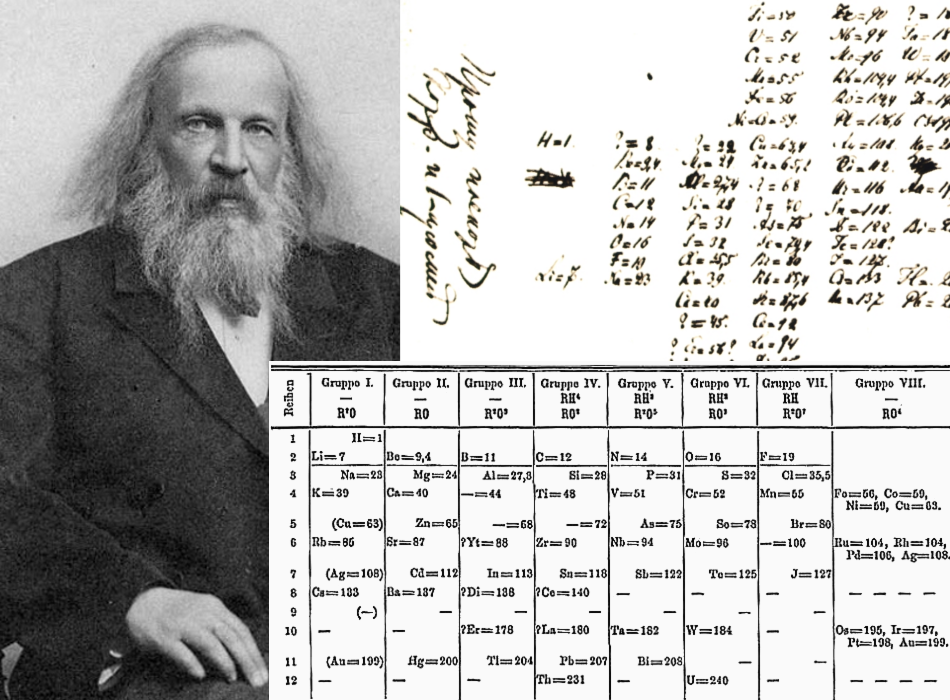
The Periodic System between Chemistry and Physics ChemistryViews
Figure 2.5.1 2.5. 1: Mendeleev's Periodic Table, as Published in the German Journal Annalen der Chemie und Pharmacie in 1872. The column headings "Reihen" and "Gruppe" are German for "row" and "group.". Formulas indicate the type of compounds formed by each group, with "R" standing for "any element" and superscripts.

Dmitri Mendeleev Inventor of the periodic table of elements New Scientist
Mendeleev was hardly the first to arrive at a periodic system. The observation that certain types of elements prefer to combine with certain other types prompted early chemists to classify the elements in tables of chemical affinities. In 1817 the German chemist Johann Wolfgang Döbereiner noticed the existence of groupings of elements in.